German Working Group for COVID Vaccine Analysis - SUMMARY OF PRELIMINARY FINDINGS - July 6, 2022
06.07.2022 Working Group for COVID Vaccine Analysis
This summary is a preliminary, continuously evolving presentation of our research and findings on the so-called COVID-19 vaccines, as well as the effects we found on the human body and the blood in particular. The summary is intended for the public interest and to encourage further scientific discussion.
Legal Information
Responsible in terms of the German Press Law are:
Arbeitsgruppe Impfstoffe Aufklärung, Expertcouncil.one e.V. (i. G.),
agimpfstoffeaufklaerung@protonmail.com
Responsible Editor: Dr. rer. nat. Klaus Retzlaff, Böklinger Straße 36, 39444 Hecklingen Akademie für Gesundheit Sport und Kommunikation e.V., agskev@protonmail.com
Conuvive, Repräsentant Deutschland Holger Reißner (european industrial engineer) Stiftung Ärzte für Aufklärung Hamburg, kontakt@aerzte-fuer-aufklaerung.de
The rights to all images, graphics and photos are owned by our authors unless otherwise stated.
The COVID-19 vaccination programmes must be stopped immediately
The German Working Group for COVID Vaccine Analysis has made its initial findings publicly available in a wide-ranging report:
1. Toxic substances were found in all of the samples of COVID-19 vaccines - without exception.
2. The blood samples of all the people who had been vaccinated showed marked changes.
3. The greater the stability of the envelope of lipid nanoparticles, the more frequent are vaccine side effects.
1. Electron microscope image of dried vaccine and X-ray spectroscopy result
2. Dark-field microscope image of the blood sample of a vaccinated person
3. Vaccination side effects and (decreasing towards the right) stability of the lipid nanoparticle envelope
1. In all samples of COVID-19 vaccines, without exception, components were found, using several methods of measurement, that:
- are, in the quantities found, toxic according to medical guidelines,
- had not been declared by the manufacturers as present in the vaccines,
- are for the most part metallic,
- are visible under the dark-field microscope as distinctive and complex structures of different sizes,
- can only partially be explained as a result of crystallisation or decomposition processes,
- cannot be explained as contamination from the manufacturing process.
2. The comparison of blood samples from unvaccinated and vaccinated individuals by means of dark-field microscopy showed noticeable changes in the blood of each person who had been vaccinated with the COVID-19 vaccines. This was evident even if those people hadn’t at that point displayed any visible reaction to the vaccinations. Complex structures similar to those in the vaccines were found in the blood samples of the vaccinated. Using artificial intelligence (AI) image analysis, the difference between the blood of vaccinated and unvaccinated people was confirmed.
3. The stability of the lipid nanoparticle envelope is closely correlated with the incidence of vaccine side effects and injury. The more stable this envelope, the greater the amount of mRNA that penetrates cells, where the production of spike proteins then takes place. These results correspond with the findings of pathologists who have carried out autopsies on people who died due to vaccine injury. Spike proteins were detected in damaged tissue. Researchers suspect that the spike protein is, in itself, toxic.
The German Working Group for COVID Vaccine Analysis is an interdisciplinary working group that has undertaken the task of analysing the contents and the effects of the novel COVID-19 vaccines. The group consists of independent scientists, including physicians, physicists, chemists, microbiologists, pharmacologists and alternative health practitioners, supported by lawyers, psychologists, analysts and journalists. The Working Group for COVID Vaccine Analysis uses modern medical and physical measuring techniques, the results of which have confirmed and complemented each other: Scanning Electron Microscopy (SEM), Energy Dispersive X-ray Spectroscopy (EDX), Mass Spectroscopy (MS), Inductively Coupled Plasma Analysis (ICP), Bright Field Microscopy (BFM), Dark Field Microscopy (DFM) and Live Blood Image Diagnostics, as well as analysis of images using Artificial Intelligence. The Working Group for COVID Vaccine Analysis continues to work in close cooperation with several international groups that are carrying out similar investigations and who have obtained results consistent with our own. The results from our analysis of the vaccines can, consequently, be regarded as cross-validated. There are questions that need to be satisfactorily answered by the vaccine manufacturers and, in Germany, by the Paul Ehrlich Institute (the agency of the German Federal Ministry of Health responsible for the regulation of vaccines in that country). Possible causal links between the vaccines and fatalities need to be investigated.
In order to avert a direct and imminent danger to human life and public safety, we ask that the COVID-19 vaccination programmes be discontinued immediately.
The Working Group for COVID Vaccine Analysis
Contact: agimpfstoffeaufklaerung@protonmail.com
SUMMARY OF PRELIMINARY FINDINGS
WORKING GROUP FOR COVID VACCINE ANALYSIS
FOREWORD
We are an internationally networked working group, with a core team of more than 60 doctors, physicians, pharmacists, scientists, mathematicians, alternative health practitioners, lawyers and journalists. We have pooled our skills and technical expertise to help shed light on what we believe to be the largest pharmacological experiment ever carried out on the human race. Never in the history of science and medicine has anyone before dared to subject an entire population, an almost entire species, to a medical – not to mention a genetic - experiment. If this kind of experiment had been proposed for any other species it would most likely have been rejected with the explanation that it violated the principle of the Species Conservation.
The fact that this experiment continues to be carried out with no sign of being stopped; the fact that there has been an unprecedented number of adverse reactions and injuries from these so-called vaccines; the fact that national statistics around the World demonstrate an unmistakeable excess mortality in the wake of the respective vaccination programmes; the fact that no public prosecutor's office has yet intervened in this matter, although the deadly effects of these programmes are already obvious; the fact that critics of the programmes have been publicly defamed, ostracised and economically ruined – all of this makes us shudder. This is why, contrary to the customary practice in science, we have decided to protect ourselves by remaining anonymous as authors of this report.
Many of our findings are still preliminary. The investigations should and must be critically discussed, digested and further refined. Much still remains to be analysed, but what we have found - we are convinced - is so important that the public in general and the scientific community in particular must be informed about it. There needs to be a wider understanding of the dangers that the COVID-19 vaccines pose to health and research into how the worst effects of these vaccines can be prevented, or at least mitigated.
We have established that the COVID-19 vaccines consistently contain, in addition to contaminants, substances the purpose of which we are unable to determine. Some of these ingredients uncovered by us have not even been listed as ingredients by the Vaccine manufacturers. Using a small sample of live blood analyses from both vaccinated and unvaccinated individuals, we have determined that artificial intelligence (AI) can distinguish with 100% reliability between the blood of the vaccinated and the unvaccinated. This indicates that the COVID-19 vaccines can effect long-term changes in the composition of the blood of the person vaccinated without that person being aware of these changes.
We have found crystalline formations in the blood of all the vaccinated people whose blood we examined. We are continuing to analyse these formations. In every case that we have examined, we have observed a rouleaux formation of the erythrocytes (the red blood cells) and we frequently observe an unusually rapid disintegration of the different types of cells in the vaccinated blood that we examine. We are exploring the hypothesis that arteriosclerosis could be just one of many long-term effects that those vaccinated with the COVID-19 vaccines will have to face. If this hypothesis is confirmed, the consequences would be wide-reaching in terms of both health and socio-economics. Autoimmune diseases weakened immune systems, inflammatory conditions, arteriosclerosis etc. are insidious diseases that can lead to organ damage, high blood pressure, heart attacks, neurodegenerative diseases, cancer, and shorten life expectancy. The unclear disease pattern conceals the possibility that COVID-19 vaccinations may play a causal role in the disease.
At the very least, each one of our findings should result in the immediate suspension of the COVID-19 vaccination programmes in order to exclude the vaccines from reasonable suspicion.
In fact, in view of the risks that we are already aware of the COVID-19 vaccination programmes should have been stopped long ago.
If we are still to achieve this goal, our findings will need to gain a high level of coverage by the media.
German Working Group for COVID Vaccine Analysis
Introduction
The summary of the results of our research presents and substantiates our findings in a way that is intended to be readily understandable. The technical reports can be found in the appendices. We have chosen this format because our foremost aim is to reach the awareness of the general public with the danger of these vaccines to human health.
Due to the unprecedented political situation that we have found ourselves in since March 2020, the contents of these reports have not been subjected to the customary peer-review process. Several highly qualified colleagues in our international network have taken a critical look at our presentations and provided feedback, for which we should like to thank them.
The analyses were carried out in different institutions in several different countries and using a variety of different methods.
The starting point was microscopic observations of the contents of the vaccines and the blood of vaccinated and unvaccinated test persons by means of various photo-microscopic methods, such as transmitted light microscopy, phase contrast microscopy and dark field microscopy. All of these methods showed inadmissibly large objects according to the rules of good manufacturing practice (GMP) in various batches and from various manufacturers in the two-digit micrometre range. Sizes of no greater than 0.22 μm are normally permissible.
For the observation of the objects in the vaccines and in the blood under the light microscope, darkfield microscopy1 is particularly suitable. An image observed in the dark-field microscope, is notable for its very high contrast. Due to the method of illumination in the dark-field microscope, the objects appear against a dark background, so light-reflecting objects can still be observed that are smaller than the optical resolution. For this reason, larger lipid nanoparticles can be seen in the dark-field microscope as bright dots - similar to the stars in the sky. Even more so, large and optically resolvable objects appear significantly more high-contrast and structured than in the phase-contrast or transmitted-light microscope.
To determine what is seen in the context of light microscopy, common standard analytical methods of various kinds have been used and continue to be used.
As a precautionary measure, we should like to point out that the analysis of vaccines, their batches and the releases (in Germany) are the responsibility of the Paul Ehrlich Institute (PEI), a government agency that is the competent authority for this task. Based on an ever-growing number of instances, it is apparent that the PEI is simply not fulfilling its duty either adequately or with the necessary transparency. The general public has very little awareness that this indeed is the case. This dereliction of duty by the responsible body, was the reason why we as independent scientists decided to come together to analyse samples from these vaccines.
Our summary is being constantly revised and supplemented with the results of these analyses but is not limited to the analysis of vaccines.
1 This term refers to the physical method of the optical technique, and not to the possible problematic - because scientifically not validated - interpretations of dark field observations. The technique of dark field illumination has many applications in physics and other fields. An outstanding example is the Millikan experiment, in which the dark field technique enables the observation of oil droplets in a condenser. Robert Andrews Millikan was awarded the Nobel Prize in Physics in 1923 for determining the elementary charge of an electron by means of this experiment.
Overview
1. Comparative observations using dark-field microscopy of the blood of both subjects who were vaccinated with the COVID-19 vaccines and subjects who were not: Over the course of many years, well over 10,000 structured blood analyses have been carried out. In the context of COVID-19 vaccinations, 48 vaccinated patients were examined. Without exception, all of these patients showed peculiarities that could not be observed in a single case in unvaccinated subjects. In addition, the blood of the vaccinated subjects regularly displays phenomena that have similarities with pathogenic, and often highly pathogenic, clinical presentations. These observations have also been recognised by other observers or therapists working with dark field microscopy and were, in fact, the main reason for establishing this working group to investigate the question: What is the truth about gene-based vaccines?
2. Metallic particles in COVID-19 vaccines: Investigation of metallic contaminants in vector- and mRNA based COVID-19 vaccines - Preliminary results - (Vials of COVID-19 vaccines from BioNTech/Pfizer, Moderna and Astra Zeneca) are investigated by scanning electron microscopy (SEM) and corresponding energy dispersive X-ray spectroscopy (EDX) to identify possible contaminants. Metallic particles have been found.
3. Preliminary results of standard analyses of Covid vaccines: Increase in the number of side effects due to more stable nanolipids, crystallisation effects due to decomposition of the nanolipids (e.g., cholesterol), metallic impurities in the ppm range, unspecified impurities due to calcium and aluminium. Methods: SEM EDS, mass spectroscopy (MS).
4. Antimony in Moderna: Naturally occurring antimony is highly toxic; antimony was detected in the Moderna vaccine during an inorganic analysis of the vaccines. Moderna and BioNTech/Pfizer vaccines were analysed, using an inductively coupled plasma (ICP) analyser, the results of all 41 investigated elements, except antimony, are below the respective detection limit (DL), DL results from the sample quantity, high DL, due to low sample quantity.
5. Preliminary assessment of antimony, various issues: Antimony is naturally occurring and highly toxic, but the concentration in Moderna should not be toxic. Valence in Moderna is unknown and antiprotozoal/antiparasitic effect, important metabolic functions could be disturbed, parenterally (injection) administered pentavalent sodium stibogluconate as it most likely occurs in valence in Moderna, could cause side effects such as nausea, vomiting, myalgia, headache, lethargy and ECG changes, with prolonged administration pneumonia, blood count damage and liver dysfunction, serious damage occurs mainly in the liver and heart. These are all clinical events to be observed following vaccination.
6. Video documentation: In the video of the vaccines, a Zeiss Axiolab microscope was used to examine various batches of the Comirnaty vaccine from BioNTech/Pfizer. At the end of the video, some blood samples from people who had been vaccinated can also be seen, in which structures similar to those in the vaccines were found. By way of comparison, two influenza vaccines were examined in another video. The differences are clearly visible. The videos can be found here: https://t.me/agimpfstoffe.
7. Is there a change in the blood as a result of COVID-19 vaccinations? Comparative analyses based on image analysis using artificial intelligence. A primary attempt to present the blood of vaccinated and unvaccinated people indicates that there are changes in the blood. This is illustrated by artificial intelligence (AI) trained using the images of the blood of the vaccinated and unvaccinated. Whether this will continue to be the case, or for how long after vaccination, remains unknown; as of 1.5.2022, the sample size is still too small for this. Because the changes have been shown in every case in this small sample, and because of the potential significance of the fact that under certain circumstances a human organ could be changed by COVID-19 vaccination, we therefore appeal to others in the scientific community to check the results before we publish the definitive results.
Comparative Observations of the Blood of Subjects Vaccinated with COVID-19 Vaccines and those Unvaccinated
Dark-field microscopic blood analysis is a proven and evidence-based procedure in the field of complementary and alternative medicine. High-resolution microscopes with a specialised dark-field oil condenser are used.
In practice: 12 years of experience in dark field microscopic blood screening with over 10,000 people from all over the world, including people who were near the attack in New York on 9/11/2001, as well as in a cancer clinic with people from Australia, USA, Canada and Europe.
How is the analysis conducted and what happens during the blood examination with the darkfield microscope?
Dark-field microscopy is a qualitative method for the analysis of blood. It originated from the research of the zoologist and bacteriologist Prof. Dr. Enderlein (1872-1968), who used this technique intensively to study blood and its components. Today, dark-field microscopy is used around the world as a scientific and evidence-based method. The live blood is analysed directly and immediately. The quality of the blood cells in terms of shape, size and elasticity can be observed and their distribution in the extracellular fluid can be examined. In addition, indications of bacterial and parasitic contamination can be detected. The decay and degradation process is also analysed. With this technique, fluid and nutritional intake can also be represented indirectly.
The life cycle of the blood components on the slide is observed, analysed and evaluated over time until final decomposition.
Generally applicable standards for the performance of the dark-field blood examination: The examination conditions should always be carried out according to the same standards. The patient should appear for the examination fasting (abstinence from food for at least 8 hours). 1 glass of water (200 ml) should be drunk before the examination. The examination room should be free of disruptions, low in radiation and absolutely hygienically clean, without, for example, a mobile phone as a possible source of interference. First of all, the hands are cleaned under running water (without soap). Then, using a lancing device, the fingertip is carefully punctured to obtain a drop of blood (capillary blood), whereby the first drop is wiped off the finger with a swab. Two drops of blood are carefully applied to the slide (the drop of blood should "jump" onto the slide) to avoid mechanical damage to the blood cells. This allows the blood to be optimally transferred to the slide. Cover slips are then used to very carefully cover the blood sample from a low distance from the slide.
Analysis of the blood:
The slide with the drop of blood is immediately analysed under the darkfield microscope. With 100x magnification, an approximate overview of the following is possible:
• Ratio of blood cells to blood plasma
• Shape and number of the various blood cells
• Mobility and activity of the blood cells
With 1,000x magnification, the condition of the blood can be evaluated Sometimes 400x magnification is used as well.
The results are documented.
After 1 and 2 hours and at further intervals, the blood is observed on the slide:
• Shape of the blood cells
• Activity of the blood cells
The monitoring procedure continues until the blood components have completely disintegrated.
One can see the movement of the blood cells as well as the shape and stability of the cell membranes (up to the point of degradation), as well as the activity and survival time (from a few hours to 6 weeks) of the blood cells and various pathological features of the extracellular fluid.
For many years, we have observed an increase in the burden of both light and heavy metals in the blood of patients in our practices. This can be proven and confirmed by provocation tests from environmental laboratories and from spectrophotometric measurements.
The blood of healthy unvaccinated subjects is characterised by a slight Brownian molecular movement, by a clear harmonious distribution of blood cells and by a clear extracellular matrix (Figure 1).
Figure 1: Blood of a healthy unvaccinated volunteer. You can see (a) relatively uniformly shaped erythrocytes (red blood cells), (b) two neutrophils and (c) a basophilic granulocyte (white blood cells).
The blood from vaccinated patients (specifically those having had either the BioNTech/Pfizer or the Moderna vaccines) stands out mainly because of the following differences:
• Novel structures (Figures 3, 4 and 5) that we have previously only seen in the vaccines themselves directly (sealed vaccines were analysed, at least 3 batches with over 12 vials), e.g. rectangular and square crystal shapes, spirals, etc., these kinds of structures have never been found in human blood before. These structures were most frequently found in the Comirnaty vaccine from BioNTech/Pfizer (size up to 25 μm, erythrocyte has approx. 7.5 μm diameter) (Figure 4, 2nd row left).
• There is a clear deformation of the cell membranes of erythrocytes, which we otherwise only encounter in chronically ill people and people with severe degenerative diseases (Figures 4, 2nd row left).
• Blood clots, lamellar structures that can occlude small vessels (size up to 40 μm), are also frequently seen. The blood viscosity (reduced flow capacity of the blood) in vaccinated people is significantly elevated. We normally only see this evidence in people who are at risk of stroke or thrombosis (Figures 4, 2nd row left).
• Observation of the decomposition process (blood from healthy people can live actively on the slide for days) shows a rapid progression, the blood sometimes only lives for a few hours. • However, the decay processes of erythrocytes, so-called ghosts/erythrocyte shadows, can also be seen at the beginning, which we normally only see in patients with severe chronic inflammatory processes and chronically ill patients (Figures 2 to 5).
• What is striking is that one does not necessarily have to know whether the patient has been vaccinated or not. This can be recognised by the conspicuous changes in the patient's blood.
From our experience with numerous patients, we should like to report that there are clearly significant dissimilarities in the blood of vaccinated and unvaccinated people. The microscope does not lie. Clean work, a great deal of experience and the identical conditions or standards when taking blood samples are the prerequisites for these differences to be clearly detected.
The images of the blood of vaccinated people are very worrying, particularly because no one knows to where the body is transferring these structures. It is known from environmental medicine that heavy metals, for example, can enter the connective tissue and the brain. In the case of vaccines and the technology that they use, we are forced to conclude that these substances can spread throughout the whole organism.
Pictorial Documentation
Figure 2: The two images show anomalous objects in the blood of a 52-year-old male subject vaccinated with Comirnaty. The subject complained of severe fatigue and exhaustion.
The picture shows a similar object in unadulterated Comirnaty from BioNTech/Pfizer (from different batches). These kinds of objects are by no means unique and have been observed repeatedly by numerous independent observers in different blood samples and in samples of vaccines, so it is extremely improbable that they are the consequence of subsequent contamination. Needless to say, because of their size, such large objects can lead to disruptions in the blood circulation in the vessels.
Figure 3: Comparison of crystals in the blood and in the vaccine; on the left, crystalline formations are found in the blood of test subjects vaccinated with Comirnaty (BioNTech/Pfizer), the images on the right show that these types of crystals are also found in Comirnaty vaccines. It should be noted, however, that the amount of a vaccine dose would be insufficient to cause the high incidence in the blood that can be observed on a regular basis. It is therefore reasonable to assume that the vaccine also affects organ functions, e.g., liver function, which would result in the presence of these structures.
Figure 4: These 4 images illustrate the variety of unusual phenomena and objects found in the blood of subjects vaccinated with Comirnaty (BioNTech/Pfizer).
Figure 5: Objects that do not belong in the blood also appear in the blood of test persons who were vaccinated with Astra Zeneca. The images on the left and in the middle show the blood at 1,000x magnification, the image on the right at 100x magnification.
Figure 6: Anomalous objects in Johnson & Johnson's Janssen vector vaccine. It should be noted that objects of this type were not found in all of the samples.
Figure 7: The Comirnaty vaccine from BioNTech/Pfizer exhibits a diversity and large number of unusual objects. The vast number of crystalline platelets and shapes can hardly be interpreted as impurities. They appear regularly and in large numbers in all samples.
Figure 8: It is probable that these objects in Comirnaty (BioNTech/Pfizer) are impurities, the origin of which needs to be clarified. The size exceeds 50 µm in some cases.
Figure 9: This image of the tissue of a vaccinated person's lung reveals a birefringent particle. These types of particles are considered foreign to the human body. Note the striking similarity to the object in Figure 8, bottom left. [Source: Courtesy of Arne Burkhardt and colleagues, 2022].
Metallic particles in COVID-19
Using modern observation techniques and physical analysis procedures, such as
• Scanning Electron Microscopy (SEM)
• Energy Dispersive X-ray spectroscopy (EDX)
the COVID-19 vaccine doses from
• AstraZeneca
• BioNTech/Pfizer
• Moderna
• Johnson & Johnson
• Lubecavax
• Influspit Tera
were investigated.
The following predominantly metallic elements were unexpectedly detected in the doses from AstraZeneca, BioNTech/Pfizer and Moderna:
• Alkali metals: caesium (Cs), potassium (K),
• Alkaline earth metals: calcium (Ca), barium (Ba),
• transition metals: cobalt (Co), iron (Fe), chromium (Cr), titanium (Ti),
• Rare earth metals: cerium (Ce), gadolinium (Gd),
• Mining group/metal: aluminium (Al),
• Carbon group: silicon (Si) (partly support material/slide),
• Oxygen group: sulphur (S)
The background to our investigations
In late summer 2021, metallic contaminants were found in Moderna vaccine vials in Japan. As a result, the Japanese authorities suspended the use of three Moderna batches containing 1.63 million doses [1]. The deaths of two men aged between 30 and 40 who died within days of receiving Moderna COVID-19 vaccine from the batches in question, which contained contaminants, can also be viewed in connection with this [2].
Also, a few weeks later, white floating matter was found in two unused vials of Pfizer COVID-19 vaccine [3]. Recently, Moderna had to recall 764,900 doses of its COVID-19 vaccine in Europe after contamination was found in a vial [4]. Prompted by the various findings of foreign contamination in Japan, several vials of mRNA-based COVID-19 vaccines (BioNTech/Pfizer and Moderna) and AstraZeneca were examined using scanning electron microscopy (SEM) and irradiated with an appropriate amount of energy to detect or rule out possible contamination using dispersive X-ray spectroscopy (EDX). SEM provides detailed high-resolution images of a sample of interest using a focused beam of high-energy electrons and generates low-energy secondary electrons. The intensity of these secondary electrons is mainly determined by the surface topology of the sample. In addition, X-rays are generated by the interaction of the sample with the primary electron beam.
These can be used by means of energy dispersive X-ray spectroscopy (EDX) to obtain element-specific information.
Among the metallic particles that were detected are cobalt (Co), iron (Fe), chromium (Cr), titanium (Ti)), rare earth metals like e.g. cerium (Ce) and gadolinium (Gd), barium (Ba), caesium (Cs), aluminium (Al), but also silicon (Si), sulphur (S), potassium (K) and calcium (Ca). The size of the particles varied from 1 µm to 100 µm. However, further confirmation and measurements are needed and are planned for the near future. A more detailed report of these preliminary findings is available as well and is available for download [5].
SEM and EDX - one example of many ...
The figure (left) shows an image obtained with a secondary electron microscope (SEM) of a contaminant found in an AstraZeneca sample (Vaxzevria: lot 210101) together with an EDX point spectrum (right) recorded at the location outlined in blue. The EDX dot spectrum shows the presence of silver (Ag) as well as traces of sulphur (S), cobalt (Co), cerium (Ce) and gadolinium (Gd) in this contamination. The other point scans recorded provide similar results. The surrounding material is the organic part of the vector vaccine.
Interpretation from a medical point of view
In stable form, caesium (Cs) is of little toxicological significance. Absorbed caesium behaves in a similar way to potassium (K), so there may be a risk to the balance between potassium in the cell and in the blood. Potassium deficiency can lead to cardiac arrhythmias and developmental disorders. Carcinogenic effects are associated with radiocesium. But here too, as with the other elements found, it is unclear which isotopes of the elements in question are actually present.
Hypothesis: From a medical point of view, caesium has no therapeutic value; on the contrary, one would have to assume that the addition of caesium disturbs the potassium balance and could cause vital cells (e.g. defence cells) to die in order to possibly accelerate the effect of the vaccination or to avoid endangering that effect.
Potassium (K) is a vital mineral. It facilitates the transmission of electrical signals between cells through the activation of specific enzymes. Potassium plays a decisive role in regulating the pH value and is also involved in the regulation of blood pressure. Potassium deficiency can exacerbate high blood pressure. Potassium intoxication has the potential to be life-threatening; symptoms include muscle weakness, paralysis and cardiac arrhythmias. It is not known whether the intended purpose of increasing the pH is to provide the vaccine with better access to the cell at the target site.
Calcium (Ca) is a very important mineral. Indeed, this mineral is the most important in the human body in quantitative terms. Calcium keeps bones and teeth strong and is an essential factor in blood clotting. Every cell in the body needs calcium. Calcium stabilises cell walls, is essential for signal transmission in the cell and for the transmission of signals in the nervous system (e.g. hearing, seeing, touching) and is also necessary for the functioning of the muscles. In the long run, an elevated calcium concentration can lead to urinary stones, impaired kidney function, risk of heart disease and prostate cancer. Typical symptoms are increased water excretion, nausea, constipation, vomiting, sometimes pancreatitis, cardiac arrhythmias, listlessness, muscle weakness, extreme drowsiness, psychosis and, in extreme cases, coma.
Barium (Ba) is toxic to humans and animals in soluble form. It disrupts the potassium balance. In high concentrations, barium blocks the passive potassium channels in the cell membrane. This leads, for example, to a disturbed function of the muscle cells and to potassium deficiency in the blood, as potassium remains in cells in increased amounts. Barium is also used in dentistry. This application is not without problems unless the barium that is bound to the dental cements has a high adhesion constant and enters the blood as a freely soluble compound.
Cobalt (Co) is a heavy metal. Cobalt has a beneficial action in combination with vitamin B12 on the human body and is essential for life. In the event of an overdose, however, symptoms such as nausea, nausea, visual disturbances, heart problems and damage to the thyroid gland can occur.
Iron (Fe) serves an important function in transporting oxygen in red blood cells. Depending on the dose, iron can be toxic to the gastrointestinal tract, the cardiovascular system and the central nervous system. In acute iron poisoning, symptoms such as vomiting, diarrhoea, coma and bleeding in the gastrointestinal tract occur, later (up to 24 h) fever, blood clotting disorders, liver and kidney damage. Iron overload can cause long term damage by depositing iron in organs and thereby pose a risk for liver diseases (cirrhosis, cancer), cardiac insufficiency, diabetes mellitus or arthrosis, secondary haemochromatoses.
Chromium (Cr) is not toxic in combination. Free and suspended chromium can lead to acute symptoms of poisoning, including anaemia, platelet deficiency, tissue death, especially in the kidneys, gastrointestinal inflammation. Chronic exposure to chromium triggers diseases such as allergic asthma, bronchitis, skin inflammation, conjunctivitis and liver inflammation.
Titanium (Ti): Titanium alloys are used in surgical instruments as well as implants, such as bone and joint replacements, dental implants, jaw and facial treatments, cardiovascular devices, etc. Titanium dioxide is used as a whitening agent in cosmetics, food and nutritional supplements, medicines and more. Currently, its safety is being re-evaluated and has increasingly been linked to genotoxicity2.
Cerium (Ce) Cerium is of low toxicity. Direct contact may cause itching, heat sensitivity and skin lesions.
Gadolinium (Gd) is used as a contrast agent in magnetic resonance imaging. In the meantime, there is increasing evidence of residues of the metal gadolinium in the brain. The official recommendation is to use gadolinium-containing contrast media only in unavoidable examinations for now, also because acute to chronic kidney diseases have now been associated with gadolinium-containing contrast media. The long-term risks of gadolinium contrast agent administration are still unknown. Free gadolinium is highly toxic, and free gadolinium accumulates in bone. It is possible that gadolinium may facilitate the crossing of the blood-brain barrier.
Aluminium (Al) is the most common metal in the earth's crust and an important material for a wide variety of applications such as packaging material, food additives, cosmetics, and medicines. Aluminium is sometimes even added to drinking water. In humans, aluminium has been suspected of contributing to Alzheimer's disease [6]. In vaccines, aluminium is believed to be used as an adjuvant [7].
Silicon (Si) is the second most abundant element by mass in the biosphere after oxygen. Silicon is a building block for connective tissue, skin, tendons and ligaments, bones and cartilage. Silicon is important for flexibility and elasticity in the vessels, e.g., in the cardiovascular system. It is also important for the immune system, e.g. for the production of lymphocytes and "scavenger cells" in the fight against microorganisms. An excess of silicon can result in the dissolution of blood cells and can cause anaemia. Long-term intake of excessive amounts of silicon can lead to urinary stones. Silicon in the form of a food supplement should not be taken during pregnancy. Silicon absorbed from the air in higher concentrations can lead to the lung disease silicosis. The silicon in the samples probably comes from the carrier material.
2 https://www.bfr.bund.de/cm/343/neubewertung-von-titandioxid-bfr-zieht-aehnliche-schluesse-wie-die europaeische-behoerde-fuer-lebensmittelsicherheit.pdf
Sulphur (S) is an important component of several protein building blocks (amino acids). Sulphur plays a role in the formation and repair of cells and tissue as well as in strengthening the immune system and is important for the production of hormones and enzymes. Pure sulphur is not toxic. However, certain sulphur compounds in higher doses are toxic and have, for example, inhibitory effects on enzymes or promote carcinogenesis. Known toxic sulphur compounds are, for example, hydrogen sulphide, sulphur dioxide, sulphuric acid and carbon disulphide. Acute poisoning leads to states of agitation, unconsciousness and respiratory paralysis. In chronic poisoning, sleep disturbances, irritability, visual disturbances, weight loss and kidney damage occur. It has not yet been determined in which combination sulphur occurs in the vaccines and whether they are harmful or toxic.
References
[1] https://www.reuters.com/business/healthcare-pharmaceuticals/japan-finds-stainless-steel-particles suspended-doses-moderna-vaccine-2021-09-01/
[2] https://www.forbes.com/sites/graisondangor/2021/08/28/two-men-in-japan-die-after-covid-19-shots from-supply-suspected-of-contamination/?sh=2a03771075e4
[3] https://www.japantimes.co.jp/news/2021/09/15/national/contaminants-pfizer-tokyo-osaka/
[4] https://www.reuters.com/business/healthcare-pharmaceuticals/moderna-recalls-thousands-covid-vaccine doses-2022-04-08/
[5] https://2020news.de/moeglicherweise-toedliche-mrna-impfstoffchargen-enthalten-metallische verunreinigungen/
[6] https://www.br.de/wissen/gesundheit/aluminium-gefaehrlich-gesundheit-alzheimer-100.html [7] https://www.pei.de/SharedDocs/FAQs/DE/impfen-impfstoffe/enthalten-impfstoffe-aluminium.html
Full article attached:
Investigation of metallic contaminations found in vector- and mRNA-based COVID-19-”vaccines”
Homogeneity and inhomogeneity in the spectrum of PEG-lipid-Chain lengths and vaccine damage frequency in mRNA vaccines
The mRNA needs a protective envelope to enter the cells. This protective envelope consists of nanolipids. The nanolipids are stabilised by layers of polyethylene glycol (PEG). The PEG is formed from chains of different lengths. The structure of a nanolipid particle protecting the mRNA is shown schematically in the following figure 1.
Figure 1: Schematic structure of an archetypical nanolipid particle with mRNA on the inside. This shell protects the mRNA from decay and degradation through the body's own defence mechanisms and it ensures that the mRNA can be introduced into the body cell to reprogram the cell function to produce the spike proteins. It can cause allergies. Despite their widespread use, a toxicity study of the suspected cationic liposomal nanoparticles is yet to be conducted. The outer wavy curls indicate the chains, image source: doi:10.3390/vaccines9010065.
Figure 2: Diagram of the setup for determining the mass spectra using MALDI (Matrix Assisted Laser Desorption/Ionisation) and TOF (Time of Flight) analysis. Image source:
https://de.wikipedia.org/wiki/Matrix-unterst%C3%BCtzte_Laser-Desorption/Ionisation.
Using time-of-flight mass spectroscopy (TOF-MS), it is possible to detect PEG and measure its chain length (see Figure 2). For this purpose, sample fragments are separated by means of a laser beam, ionised and then deflected at an electric mirror. Fragments of different mass move along correspondingly longer or shorter paths and are detected separately in time. PEG molecules with different chain lengths then form a distribution in the MALDI spectrum. An example spectrum can be seen in Figure 3.
Figure 3: Original mass spectrum from a sample of batch ET1831 (BioNTech/Pfizer). This sample shows a narrow spectrum of the PEG chains.
These spectra were recorded for different batches. Different distribution patterns of the PEG masses were found, which can also be interpreted as indicating differences in the quality of the batches.
Based on the observations, it can be assumed that the PEG quality has a long-term influence on the functioning of the vaccine. Accordingly, batches with a very broad mass spectrum and longer chains would protect the mRNA less effectively. The nanolipids are more unstable and can disintegrate early and before penetrating the cell, thus releasing the mRNA outside the cell. Autohydrolysis and enzymatic processes can then destroy the mRNA and finally neutralise its genetic function.
In these types of batches, numerous lipid crystal particles can be observed in the dark field as tiny solids, the occurrence of which in the blood vessels can lead to blockages and consequent damage of many kinds, (Figure 4).
An example of a broad PEG mass distribution pattern is shown in Figure 5. This spectrum shows a flat distribution and has longer chains.
The idea that broadly distributed PEG coatings are associated with poorer quality vaccine is illustrated by Figure 6. There it can be seen that at the expense of the greater length of PEG chains, gaps appear in the surface coating, which lead to a reduction in stability.
The comparison of the batches with differently homogeneous mass spectra in connection with the number of vaccination complications detected reveals a clear correlation, Figure 7.
Figure 4: Lipid crystal particles at 1,000x magnification in the Comirnaty vaccine from BioNTech/Pfizer. Some of the crystals are in the size range of red blood cells (Ø 7-8 µm], the so-called erythrocytes and even larger.
Figure 5: Original mass spectrum from a sample of batch ET1831 (BioNTech/Pfizer). This sample shows a relatively flat and long-chain distribution of PEG in the mass spectrum.
Figure 6: Schematic structure of a defective nanolipid particle with mRNA inside. This shell cannot safely protect the mRNA from decay. The mRNA is able to escape and is subsequently destroyed rapidly due to its instability before it has penetrated into the interior of the body cell to modify cell function and induce the production of the spike proteins which are suspected of being toxic, Image source: doi:10.3390/vaccines9010065 and modified by our author.
Figure 7: From the mass spectra of samples from different batches of Comirnaty vaccine (BioNTech/Pfizer), the maximum chain lengths were compared with the number of reported vaccination complications. A clear correlation can be seen. The blue dots are associated with the BioNTech/Pfizer batch numbers analysed.
The correlation between vaccine complications and the PEG quality of the batches indicates that it is precisely the vaccine batches that are technically complex to produce and in which nanoparticles do not disintegrate that can cause the vaccine complication (definition according to EMA).
The more stable the mRNA vaccine, the more it can induce body cells to produce spike proteins, and the more the vaccine is able to do this, the higher the risk of the vaccinated person suffering vaccine injury.
Attachment: PDF with PowerPoint presentation: Preliminary Report of standard analytics on Covid Vaccines
Antimony in the Moderna –Vaccine?
The following analyses and findings should be verified by other groups, as only a small amount of sample material was available.
The findings are significant because antimony is a toxic element and it needs to be clarified what is its function in Moderna's COVID-19 vaccine or whether it is an undesirable contaminant.
Three samples from opened vials of Moderna with residues of 1-3 g liquid each and 8 opened vials of BioNTech/Pfizer with very small residues were examined. In order to be able to provide sufficient sample material for the investigation, the substance quantities were combined separately.
An inductively coupled plasma (ICP) was used for the examination. In ICP, the atoms of an element are excited to emit radiation. This radiation is detected and the content of the respective element can be measured in comparison to standard solutions.
Since only dissolved elements can be detected in the ICP measurement (dissolution of particles, platelets, filaments, etc.), nitric acid (HNO3) was added to the samples and heated (digestion).
The exact procedure and the tables of results can be found in the appendix.
Strikingly, antimony is above the detection limit, indicating a significant concentration.
Why is antimony a dominant substance in the Moderna vaccine?
The detection limit (LOD) results from the sample quantity.
In addition, specific conditions had to be considered: on the one hand, how intensive the signal of the respective element is in the ICP and, on the other hand, what the general laboratory contamination with various substances is, e.g., double-distilled Water; laboratory equipment; ICP.
The detection limit for silicon had to be set high because the laboratory frequently handles substances that contain silicon as a main component and there was therefore a risk of contamination.
Preliminary assessment of Antimony
Antimony (Sb) is a naturally occurring element and is defined as a highly toxic metal. Pentavalent antimony is considered to be the least toxic. The most dangerous form is the gaseous antimony hydride (stiban, SbH3).
Organometallic compounds of antimony have an antiprotozoal/antiparasitic effect and are used, for example, to treat the tropical parasitic disease leishmaniasis. In the meantime, pentavalent antimony (sodium stibo[V]-gluconate) is used for this purpose, on the one hand because of its higher efficacy compared to trivalent antimony, and on the other hand because of the higher toxicity of previously used trivalent antimony compounds. Antimony is also found as an antiprotozoal adjuvant in vaccines as we have known them until now.
In Moderna, our analyses found an elevated level of antimony compared to other metal elements. The dose was not toxic in the results currently available. It is not yet clear in which valence the antimony is present in the tested Moderna vaccine. Official information about the occurrence of antimony in the vaccine in general has not been published so far.
Antimony blocks SH groups in enzymes non-competitively, which explains the antiprotozoal (also antiviral) effect, among other things. However, these sulphydryl groups are also found in enzymes for protein formation or they can be parts of active centres such as coenzymes and thus be decisively disrupted in their important metabolic function by antimony. This disturbance can also affect the bone marrow with its key function as a producer of blood cells, including red blood cells (oxygen carriers), but also white blood cells (defence cells). So far, a toxic effect of antimony oxide on the erythrozoide lineage has been described.
Antimony can be used as antimony oxide in the form of nanoparticles. The official information on the structure of the nanoparticles at Moderna, however, points to a liposomal structure of the mRNA coating. There are no references to antimony.
According to official information, the composition of Moderna with regard to lipid nanoparticles is as follows:
• Polyethylene glycol (PEG) 2000 dimyristoyl glycerol (DMG)
• 1,2-distearoyl-sn-glycero-3-phosphocholine
• Cholesterol
• SM-102 (Proprietary to Moderna)
The use of nanoparticles is predicated on the principle of providing delivery vehicles for immunosuppressive compounds. The rationale for this is that the effect of mRNA active substances depends, among other things, on their ability to trigger undesired immune responses, which can weaken the effect and trigger undesired effects in the organism. For this purpose, the principle of indirect immunosuppression is used via the nanoparticles by modifying the mRNA through the exchange of uracil for N1-methyl pseudouridine and thus making it less immunogenic [1]. This prevents immunological signalling pathways. Nanoparticles can be produced as liposomes (lipid bilayer spheres) and also metal oxide nanoparticles, including antimony oxide nanoparticles.
If we consider our analyses in which antimony is detected, it can be assumed that an antiprotozoal effect is less likely to be intended here; moreover, the presence of a lipid nanomaterial is indicated as the mRNA envelope. Thus, among the currently available findings, the assumption remains that antimony serves an immunosuppressive effect [2].
It is not possible to determine the valence of the antimony in Moderna. As described above, there are differences in toxicity depending on the chemical valence: antimony oxide, as it can be applied as a nanomaterial, is present in pentavalent form.
Now, the toxicology and immunology of nanoparticles has not yet been conclusively researched; antimony oxide nanoparticles are potentially toxic to the hematopoietic series of red blood cells. Even if, on the other hand, experiments have been carried out under laboratory conditions on artificially immortalised cell series (immortalised cell series) of haematopoietic origin (stem cells of the bone marrow), where no toxicity has been shown, in our opinion the current state of knowledge is far from sufficient to exclude decisive toxic effects of antimony oxide nanoparticles in vivo (in the organism under natural conditions).
Regardless of the obvious use of antimony in Moderna, there are many unanswered questions about the handling of nanoparticles and also mRNA per se; notwithstanding this, the application of the vaccines in a field trial is taking place a billion times over in humans.
With regard to the potentially toxic consequences, it is to be noted that the following should be considered as side effects of the pentavalent sodium stibogluconate administered directly into the bloodstream, as it most likely occurs in the valence in Moderna: nausea, vomiting, myalgia, headache, lethargy and ECG changes; with prolonged administration, pneumonia, blood count damage and liver dysfunction are to be noted. When they occur, more serious damage mainly affects the liver and heart. These are all clinical events observed after vaccination.
The number of unreported cases of people who received Moderna vaccines directly in the bloodstream is probably high, since the authorities (e.g. WHO) decided years ago that aspiration before vaccine application was no longer necessary. This incomprehensible and erroneous assessment was recently revoked by the Stiko/RKI and aspiration is again recommended.
Antimony in muscle tissue is associated with the degradation of muscle fibres, especially at the junctions of muscles to nerves. Pathological changes in spinal cord neurons caused by antimony are described in the literature [3].
It is still unclear where the components or decay products of the vaccines and the spikes produced are deposited in the body. Studies indicate that antimony oxide nanoparticles have a toxic effect on the precursors of red blood cells [4]. These results show how far toxicological and immunological research is still in its infancy. Further studies are urgently needed before a final assessment can be made. Unfortunately, these still pending studies were not waited for and the nanoparticles are already in use in the Covid vaccinations.
It is known that:
1. the uptake of lipid nanoparticles (LNPs) by the cell is toxic; if too many LNPs are taken up, then the toxicity of the cationic lipids is so strong that it leads to programmed cell death (apoptosis).
2. the affected surviving cells produce spike proteins and can be destroyed by the antibody complement system or by killer lymphocytes.
Since this process also occurs in all immune cells, the T, B and other immune cells decrease, resulting in immunosuppression. This is thus an indirect effect of the LNPs.
Experimental rats showed e.g. plasma cytosis in the lymph nodes [5]. The plasma cells penetrate the lymph nodes and spleen if too few immune cells are present.
Figure 1: The figure shows a section through the tissue of the spleen of a patient who died in close temporal relation to a COVID-19 vaccination. The tissue exhibits holes (a), which can be explained by the demise of the cells, and amyloidosis (b). Amyloidosis is the term used to describe deposits of proteins that are difficult to break down. Amyloidosis can be caused by a rearrangement of spike proteins [6].
References
[1] Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005 Aug;23(2):165-75. doi: 10.1016/j.immuni.2005.06.008. PMID: 16111635.
[2] Ngobili, T. A., & Daniele, M. A. (2016). Nanoparticles and direct immunosuppression. Experimental biology and medicine (Maywood, N.J.), 241(10), 1064–1073.
https://doi.org/10.1177/1535370216650053
[3] Sundar, S., & Chakravarty, J. (2010). Antimony toxicity. International journal of environmental research and public health, 7(12), 4267–4277. https://doi.org/10.3390/ijerph7124267 [4] Bregoli L, Chiarini F, Gambarelli A, Sighinolfi G, Gatti AM, Santi P, Martelli AM, Cocco L (2009) Toxicity of antimony trioxide nanoparticles on human hematopoietic progenitor cells and comparison to cell lines. Toxicology 262:121–129
[5] https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment report_en.pdf
[6] Powerpoint Presentation: „Autopsy and histopathology studies on adverse events and deaths due to COVID-19 vaccinations“, conducted at Reutlingen, courtesy of Professor Arne Burkhardt an colleagues, 2022
Is there a modification in the blood as a result of COVID-19 vaccinations? - An attempt at a comparative study based on image analysis using artificial intelligence.
In recent years, algorithms that can learn autonomously from data have become part of our society's experience of computing. Applications such as autonomous driving, reasoning, text summarisation, speech recognition, image analysis and language translation have been made available to the general public.
In the course of the analysis, a tried and tested system for image analysis was used (Google AutoML Vision) to classify images. Determinations of this kind are also very accurate for classifying images of different types of clouds, for example. The system is presented with a few images of each image of a cloud with its respective classification and is trained to find commonalities between these photos. The system learns the commonalities and differences and "understands" which part is to be learned. If you subsequently attempt to classify a new image that the system has never seen before, the chance for a correct prediction is very high (more than 95%).
The same type of classification has been used in this analysis to predict whether a picture of blood is from a vaccinated or unvaccinated person. Images of professionally collected blood samples, which were then taken with the microscope camera, were used as input. Each image was given the classification "vaccinated" or "unvaccinated" and passed to the system for training.
Figure 1: Image gallery, blood from vaccinated and unvaccinated test persons. Abnormal objects are regularly found in the blood of vaccinated test persons. Do these objects trigger the classification?
In this set, 12 pictures of vaccinated and 14 of unvaccinated persons were included. Two photos from each category were not submitted to the AI for classification by the model. The first step is a training phase, which generates a model as output that can be evaluated and used to analyse subsequent images.
After the training phase, two images, one from each category, are selected to make a prediction. These images are excluded from the training and have not been analysed before.
Each model includes a positive predictive value (Precision) and a sensitivity (Recall), which refer to images of blood that were classified as false-vaccinated or false-unvaccinated. False-vaccinated results are those where the image of the blood of an unvaccinated person is classified as vaccinated. False-unvaccinated are those where the image of the blood of a vaccinated person is classified as unvaccinated. In the model trained here, no results in the false-vaccinated or false-unvaccinated category are observed.
A more straightforward approach is the "Confusion matrix", which also displays the same classification results, in that the prediction ("Predicted Label") exactly coincides with the classification ("True Label").
Figure 2: Results after training the AI, more precisely, an artificial neural network. The 4-field matrix quantifies the relative frequency of the error classes: true-unvaccinated, false-unvaccinated, true-vaccinated, false-vaccinated. Currently, this trained AI classifies with 100% accuracy, based on a very small population.
Below we show examples of quantitative predication of both the unvaccinated (Figures 3 and 4) and the vaccinated (Figures 5 and 6). The values are always close to 1, i.e. the AI classifies the example images with a high degree of probability as the blood of vaccinated and blood of unvaccinated subjects.
Figure 3: The AI correctly classifies the blood of an unvaccinated subject.
Figure 4: Here, too, the blood of an unvaccinated subject is correctly classified. It can be seen from the example that very different images, i.e. images created under different imaging conditions, are correctly classified.
Figure 5: The blood of vaccinated subjects regularly contains unusual objects that do not belong in the blood, but also the phenomenon of rouleaux. These might be the explanation for the excellent classification results.
Figure 6: It should be evident that these types of objects are capable of obstructing capillary vessels.
Evidence of alterations in the blood - regardless of the method of blood analysis - as a resulting from the COVID-19 vaccinations is increasing at an alarming rate and is being increasingly reflected in the relevant statistics.
As can be seen from the classification of vaccinated and unvaccinated, an accurate prediction can be made with a probability of more than 98%. Although this is a small sample size study, it suggests that a more extensive investigation is warranted. In particular, since this type of investigation was not done during the experimental stages and blood is a good predictor of future disease, large-scale blood testing to distinguish between vaccinated and unvaccinated individuals is urgently needed.
Attachments
(Document Collection)
Summary
Different vials of mRNA-based COVID-19-”vaccines” (Biontech and Mod- erna) are investigated by means of Scanning Electron Microscopy (SEM) and corresponding Energy Dispersive X-ray Spectroscopy (EDX) to study potential contaminations. Metallic particles comprising transition metals (e.g. cobalt (Co), iron (Fe), chromium (Cr), titanium (Ti)), rare earth metals such as cerium (Ce) and gadolinium (Gd), barium (Ba), caesium (Cs), aluminium (Al), silicon (Si), sulphur (S), potassium (K) and calcium (Ca) are found. The size of the particles varies from 1µm to 100µm. In contrast, initial investigations of the contents of Johnson & Johnson (Janssen), Lubecavax, and Influspit Tetra have not so far shown signs of these kinds of contaminations and particles. However, further confirmation and measurements are necessary and are in fact planned for the near future.
Experimental section
Different samples of COVID-19-”vaccines” are investigated by SEM/EDX. In a scanning electron microscope (SEM), the sample in question is scanned by means of a narrowly focused electron beam (5- 10nm) of several thousand electron volts energy. In the studies presented here, energy of 15000 eV (15 keV) was used, and secondary electrons were used for imaging. In addition to imaging the surface of the sample at very high resolution, chemical analysis can be performed using energy dispersive X-ray spectroscopy (EDX). At the energy of 15 keV used here, a detection depth of some micrometres is achieved.
The samples were leftovers from vials which could not be used for injection anymore or vials where the cooling chain was interrupted. The samples were prepared for REM/EDX in two different ways. Firstly, a number of microscope slides were covered with a thin film of Gold (Au) for electrical conductivity required for the measurements. The vaccine samples were drawn up with syringes exactly as performed before injecting the person to be vaccinated. Then, the vaccine samples were dripped from the syringe onto the Au covered slide. The vaccine dried for several days under ambient conditions protected from any contamination, before the samples were brought into the SEM. Secondly, other lots of COVID-19-”vaccines” were prepared in a different lab. Here, the vials were opened and the samples were directly casted onto microscope slides, were the samples dried for several days under ambient conditions protected from contamination. Then, the samples were sent to the SEM-lab. Since these microscope slides were not covered with Au, the slides were covered with a thin iridium (Ir) film prior to the SEM/EDX measurements to ensure the required electrical conductivity. Note that the EDX detector used contains a carbon-based window, hence the signals from carbon and oxygen in the EDX-spectra are not entirely reliable.
Results
The following reference sample, a sample of the protein based vaccine Lubecavax, and a number of lots of novel COVID-19-”vaccines” have been studied with SEM/EDX:
• an empty microscope slide as reference
• Lubecavax (Prof. Stöcker)
• AstraZeneca (Vaxzevria): lot 210101 and lot 1423474
• Biontech-Pfizer (Cormirnaty) lot FE7011, lot FE8045, and lot 1F1010A
• Moderna (Spikevax), lot 3004217
Empty microscope slide
Figure 1 shows a SEM image of several-square-mm area of an empty microscope slide taken directly from the original packaging. The slide was covered with a thin film of Ir to ensure electrical conductivity directly before measurement The slide is homogenous with a few microscopic scratches. The EDX-map indicates also a homogenous distribution of the chemical elements identified in the EDX-spectrum, i.e. Na), Mg, Al, K, Ca, and the main component is Si (likely SiO2).
Figure 1: Top left: SEM image of an empty microscope slide. Top right: EDX map. Bottom: EDX sum spectrum of the mapped area.
Lubecavax
This sample was prepared in another lab for SEM/EDX-experiments. In this case the sample holder was covered with a thin platinum (Pt) film prior to the SEM/EDX measurements to ensure the required electrical conductivity. Figure 2 displays a typical SEM image of the protein component of Lubecavax. The EDX-point spectra show a spot with more Na and chlorine (Cl) (likely NaCl) (Spot 1) and a spot with more organic components (Spot 4). With the exception of small amounts of S and K, no other contaminants were found.
Figure 2: Top: SEM image of the dried protein component of Lubecavax vaccine. Center and bottom: EDX point spectra of dried vaccine (spots 1 and 4). Note that Pt does not belong to the sample probed by EDX due to the sample preparation described above.
AstraZeneca (Vaxzevria: lot 210101)
This sample was prepared via a syringe on a gold covered microscope slide as described in section 2. The EDX-spectrum shown in Fig.3 is typical for the dried Vaxzevria-”vaccine”, it consists of some Na und Cl (likely NaCl) and mainly of organic ingredients.
Figure 3: Top: SEM image of dried vaccine. Bottom: EDX point spectrum of dried vaccine (site 457), marked by a blue frame.
Figure 4 shows a SEM image of a contamination found in this sample. An EDX point spectrum taken at site 616 (c.f. Fig 4) reveals the presence of silver (Ag) as well as traces of S, Co, Ce and Gd localized in this contamination. The other point scans taken yield in similar results. The surrounding material is the organic part of the “mRNA-vaccine” exposed to the electron radiation.
Figure 4: Top: SEM image of a contamination. Bottom: EDX point spectrum of site 616 (marked by a blue frame).
AstraZeneca (Vaxzevria: lot 1423474)
This sample was prepared at another lab by opening the vial and then preparing the sample as described in section 2. Figure 5 shows a SEM image of a contamination found in this sample. An EDX point spectrum taken at site 616 (c.f. Fig 5) reveals the presence of Al and S, but also Ca, Fe, and Ti present in this contamination. The other point scans taken yield similar results.
Figure 5: Top: SEM image of a contamination. Bottom: EDX point spectrum of site 107 (marked by a blue frame). Note that Ir does not belong to the sample probed by EDX due to the sample preparation described in 2.
Biontech-Pfizer (Cormirnaty: lot FE7011)
This sample was prepared via a syringe on a gold covered microscope slide as described in section 2. The EDX-spectrum shown in Fig.6 is typical for the dried Cormirnaty-”vaccine”, it consists mainly of Na and Cl (likely NaCl), phosphorus (P), which may stem from some of the lipids, and organic ingredients.
Figure 6: Top: SEM image of dried vaccine including contamination in the center. Bottom: EDX point spectrum of dried vaccine (site 225), marked by a blue frame.
Figure 7 displays a SEM image of a contamination consisting of Si alone as can been seen from the corresponding EDX-spectrum.
Figure 7: Top: SEM image of dried vaccine and a Si-contamination in the centre. Bottom: EDX point spectrum of the Si (site 224), marked by a blue frame.
Figure 8 displays an Fe-containing particle found in this sample. The particles dimensions are ≈ 2.5µm × 2.0µm
Figure 8: Top: SEM image of a 2.5µm wide contamination. Bottom: EDX point spectrum of the Fe containing particle (site 237), marked by a blue frame.
Biontech-Pfizer (Cormirnaty: lot FE8045)
This sample was prepared at another lab by opening the vial and then preparing the sample as described in section 2. Figure 9 shows a SEM image of a contamination consisting mainly of Ca, traces of Si are also present.
Figure 9: Top: SEM image of a contamination of 40-50µm size. Bottom: EDX point spectrum of this contamination (site 70), marked by a blue frame. Note that Ir does not belong to the sample probed by EDX due to the sample preparation described in 2.
Figure 10 shows a SEM image of a contamination comprising a number of chemical elements. Besides Mg, Al, Si, S, K, and Ca also the 3d transition metals Ti and Fe are detected. The other point spectra recorded at this contamination yield similar results in terms of the elements detected, with partly changing stoichiometry.
Figure 10: Top: SEM image of a contamination of 25-30µm size. Bottom: EDX point spectrum of this contamination (site 96), marked by a blue frame. Note that Ir does not belong to the sample probed by EDX due to the sample preparation described in the experimental section.
Figure 11 (top left) shows a SEM image of a particle in the form of a sphere. This sphere consists mainly of Al, some Ca and traces of Fe are also present as can be seen from the element specific spatial EDX mapping results.
Figure 11: Top left: SEM image of a sphere of 3µm diameter. Top right: Element specific EDX mapping of Al. Bottom: element specific EDX mappings of Ca and Fe.
Biontech-Pfizer (Cormirnaty: lot 1F1010A)
This sample was prepared at another lab by opening the vial and then preparing the sample as described in section 2. Figure 12 shows a SEM image of a contamination consisting mainly of S. Some Fe is also present. Furthermore, traces of Na, Al, Si, and Ca are detected. The other point scans taken yield similar results, with partly changing stoichiometry.
Figure 12: Top: SEM image of a contamination of 40µm × 10µm size. Bottom: EDX point spectrum of this contamination (site 371), marked by a blue frame. Note that Ir does not belong to the sample probed by EDX due to the sample preparation described in 2.
Figure 13 shows a SEM image of a contamination comprising significant amounts of Ti, traces of Na, Al, Si, S, and Ca are also detected.
Figure 13: Top: SEM image of a particle of 5µm size. Bottom: EDX point spectrum of this particle (site 394), marked by a blue frame. Note that Ir does not belong to the sample probed by EDX due to the sample preparation described in 2.
Moderna (Spikevax: lot 3004217)
This sample was prepared via a syringe on a gold covered microscope slide as described in section 2. The EDX-spectrum shown in Fig.14 is typical for the dried Spikevax-”vaccine”, it consists of Na and Cl (likely NaCl), traces of P, which may stem from some of the lipids, and mainly organic ingredients.
Figure 14: Top: SEM image of dried vaccine. Bottom: EDX point spectrum of dried vaccine (site 68), marked by a blue frame.
This sample was prepared via a syringe on a gold covered microscope slide as described in section 2. Figure 15 shows a SEM image of a contamination comprising significant amounts of Si. Besides traces of Na, Mg, Al, P, S, Cl, and Ca also the metals Cs, Cr ,Fe , and copper (Cu) are detected.
Figure 15: Top: SEM image of a particle of 50µm size. Bottom: EDX point spectrum of this particle (site 21), marked by a blue frame.
This sample was prepared via a syringe on a gold covered microscope slide as described in section 2. Figure 16 (top left) shows a SEM image of a rod like contamination. This rod like structure consists mainly of Si, some Ca and Al are also present as can be seen from the element specific spatial EDX mapping results.
Figure 16: Top left: SEM image of a rod-like structure of 20µm length. Top right: Element specific EDX mapping of Si. Bottom: element specific EDX mappings of Ca and Al.
This sample was prepared from a vial of the same lot but at another lab by opening the vial and then preparing the sample as described in the experimental section. Figure 17 shows a SEM image of a contamination comprising significant amounts of Si, Ti, and Fe. Traces of Na, Mg, Al, K, and Ca are also detected.
Figure 17: Top: SEM image of a particle of 15-20µm size. Bottom: EDX point spectrum of this particle (site 380), marked by a blue frame. Note that Ir does not belong to the sample probed by EDX due to the sample preparation described in 2.
This sample was prepared from a vial of the same lot but at another lab by opening the vial and then preparing the sample as described in the experimental section. Figure 18 shows a SEM image of a contamination comprising significant amounts of Al, Si, S, and Ba. Traces of Na, Mg, S, Cl, K, Ca, Ce, Cr, and Fe are also detected.
Figure 18: Top: SEM image of a contamination of 20-30µm size. Bottom: EDX point spectrum of this particle (site 179), marked by a blue frame. Note that Ir does not belong to the sample probed by EDX due to the sample preparation described in 2.
Preliminary results of standard analytical tests for Covid vaccines
Summary
The major breakthrough in the production of the mRNA vaccines by Biontech/Pfizer and Moderna was the development of an envelope made of cationic lipids. Pure mRNA is easily degraded by cleaving enzymes called ribonucleases and has difficulty crossing the cell membrane. A double layer of lipid molecules forms small spheres and packaged inside them is the mRNA. Because of their small size, they are called lipid nanoparticles. In order to stabilise these nanoparticles a coating of PEG (polyethylene glycol) is needed. Leaving aside the issue of genetically manipulating the body's own cells to express S-protein antigens, the question arises as to the properties of these nanolipids and their protective PEG coatings. Are these materials themselves harmful to health and are these different properties perhaps the cause of side-effect profiles that are not dependent on the manufacturer?
The COVID-19 mRNA vaccines approved by the EMA contain parts of the genetic information of SARS CoV-2 in the form of messenger ribonucleic acid (mRNA). During administration of the vaccine, the mRNA is injected into the muscle and thereby enters the body's cells. The lipid nanoparticles are then incorporated into the body cell via endocytosis, i.e., through an invagination in the area of the cell membrane, where they can release the transported mRNA into the cytosol. In these cells, viral S-protein parts are then synthesised in the ribosomes on the basis of the coded blueprint of the mRNA and then expressed on the membrane in order to trigger a specific immune response. The mRNA itself is degraded by the body after some time, with periods of several weeks being mentioned.
To classify the vaccines, we used (A) bright-field microscopy BFM, (B) dark-field microscopy DFM, (C) scanning electron microscopy SEM, (D) energy dispersive X-ray spectroscopy EDS, (E) total reflectance X ray fluorescence spectroscopy (TXRF) and (F) time-of-flight mass spectroscopy TOF-MS.
In BFM and DFM, the formation of crystalline structures could be confirmed. A comparison with cholesterol sediment samples confirmed the hypothesis that the decay products of the nanolipid particles resemble, among other things, the "liquid crystal structures" of cholesterol. Cholesterol is incorporated into the nanolipid layer and thus facilitates endocytosis, among other things. Surface tension and static electricity influence the formation of microscopic crystals with geometries of varying complexity.
After drying, very large rectangular platelets were found using scanning electron microscopy (SEM) of Moderna samples, which turned out to be mainly NaCl crystals after EDS analysis. However, there is reason to believe that these are larger saline crystallisations of smaller lipid/cholesterol crystals. Furthermore, particulate impurities were also found using SEM, for example stainless steel metal particles (Fe-Ni-Cr). However, it is difficult to estimate whether these amounts are significant enough to pose a health risk. Additional measurements with TXRF of larger sample areas revealed no relevant spectral signals, which means that the proportion of metallic contaminants of this kind can presumably be estimated in the low ppm range. TXRF, however, showed noticeable peaks of Ca, but also small amounts of Al and Mg. However, all three elements are not listed in the manufacturers' data sheets.
The most significant result at this stage is a correlation between the polymerisation behaviour of PEG and the vaccination side-effect profile for Pfizer/Biontech. MALDI TOF-MS revealed a batch-dependent degree of polymerisation of PEG. If the maximum of the distribution of the measured PEG molecular weights is then plotted against the reported numbers of adverse reactions, a more narrowly distributed and shorter-chain coating indicates an increase in the number of vaccine adverse reactions. Consequently, a more uniform coating could increase the decay half-life and thus favour the further transportation to other tissue and organ parts. Conversely, the quantity of lipid and cholesterol crystals formed as a decay product is associated with a more unstable, more widely distributed and longer-chain PEG coating. Of course, these types of mixed crystals, when formed in narrow microcapillaries, are also potential hazards.
Further investigations of gel-filtered vaccine samples with infrared spectroscopy FTIR and micro-Raman spectroscopy confirmed the suspicions above and showed no evidence of contamination with graphene or graphene oxide.
Introduction
The global COVID-19 pandemic has given an unprecedented boost to the development of mRNA vaccines. This can be explained by the fact that, unlike conventional vaccines, RNA vaccines can be developed quickly, produced in large quantities and theoretically adapted to different pathogens. A review of RNA based drugs using methods of scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS), total reflectance X-ray spectroscopy (TXRF), infrared spectroscopy (FTIR) and mass spectrometry (MS) can only capture certain qualitative characteristics of these medical-use substances and would actually require the full spectrum of biochemical-microbiological and physical analytics.
RNA has a very low stability and is highly sensitive to the surrounding environment. The fact that DNA is stable and RNA unstable can be attributed to the different sugars in the DNA backbone. Ribose in RNA contains a hydroxyl group at the C2′ position, which destabilises the phosphodiester bond. This 2′-OH group can intramolecularly attack the phosphate group at C3′ of the nucleotide. This leads to autohydrolysis [1] of the RNA even in the absence of decomposing enzymes. In deoxyribose in the DNA backbone, this OH group is not present. Therefore, DNA does not autohydrolyse and DNA fragments can remain stable for hundreds or even thousands of years.
Figure 1: Autohydrolysis affects the stability of RNA. a) Scheme of autohydrolysis on an RNA strand with the 2′,3′-cyclic phosphate intermediate. An intramolecular nucleophilic attack of the 2′-OH group initiates cleavage of the phosphodiester. The absence of the 2′-OH group in DNA prevents cleavage there. b) Structures of the cyclic nucleoside monophosphate isomers. c) Nomenclature and structures of the RNA nucleobases (N).
The clear challenge for mRNA therapeutics is their susceptibility to nucleases, illustrated by a half-life in serum of <5 min [2]. Although chemical modifications of siRNA have been very successful in improving stability and reducing immunogenicity [3], they have not been successful for mRNA due to the sensitivity of the translational machinery to these modifications [4]. Another challenge for mRNA is the lack of cellular uptake of naked mRNA in most cell types [5], with the exception of immature dendritic cells [6]. These two challenges are solved by incorporating a nucleoside-modified or sequence-modified mRNA into a delivery system that both protects the mRNA from enzymatic attack and facilitates cellular uptake. For example, incorporation into lipid nanoparticles protects the mRNA from enzymatic attack and increases cellular uptake and expression up to 1000-fold compared to naked mRNA when administered in animal models [7,8].
The fragmentation of ions can be studied with the help of mass spectrometry. Thus, structural information about lipids and nucleic acids can be obtained. c fragments are formed by cleavage of the bond between the 5′-O and the phosphorus atom. The c-fragments of RNA dinucleotides have the same mass-to charge ratio (m/z) as the base-catalysed RNA fragments after autohydrolysis. The exact composition of Pfizer-BioNTech LNP [9] and Moderna LNP [10] has been published.
Figure 2: mRNA lipid nanoparticle structure with 1-10 mRNAs capable of being copied. The lipid polyethylene glycol (PEG) forms the surface of the lipid nanoparticle (LNP) together with DSPC, which forms a bilayer. Cholesterol and the ionisable lipid in charged and uncharged form can be distributed over the LNP.
Cationic polymers have been used quite extensively for nucleic acid delivery for many years. In the simplest application, cationic polymers are mixed in excess with nucleic acid to form electrostatically bound cationic polyplexes. Although many of these polymers have been developed, they are not as advanced as lipid nanoparticles for nucleic acid delivery, and the number of animal studies in which they have been successfully applied to vaccines is very limited. Nano-lipids are then coated with hydrophilic PEG to stabilise them in aqueous media and to limit protein and cell interactions when delivered in vivo.
ALC-0315 in combination with DSPC, cholesterol and a PEG lipid ALC-0159 is the delivery system in BioNTech's SARS-COV-2 trials [9]. The company began development of its SARS-CoV-2 vaccine with four mRNA-encoded immunogens, two of which were nucleoside-modified, one unmodified and one self-amplifying.
Disintegration of mRNA vaccines
The Moderna COVID-19 vaccine must be stored at -25 °C to -15 °C, but is also stable for up to 30 days between 2 °C and 8 °C and up to 12 hours between 8 °C and 25 °C [11]. The Pfizer/BioNTech COVID 19 vaccine is stored at -80 °C to -60 °C and can be stored if thawed at 2 °C to 8 °C for up to 5 days before dilution with saline prior to injection [12]. The dry ice temperatures required for the Pfizer vaccine are more difficult to achieve during distribution and storage than the normal freezing temperature required for the Moderna vaccine. The reasons for these temperature differences are not readily obvious, as both vaccines contain similar concentrations of sucrose as a cryoprotectant. Moderna's mRNA LNPs are frozen in two buffers, Tris and acetate [10], while Pfizer/BioNTech's vaccine uses only one phosphate buffer [9]. Phosphate buffers are known to be suboptimal for freezing as they tend to precipitate and cause abrupt pH changes at the onset of ice crystallisation [13,14].
The mechanism of RNA autohydrolysis in vitro has been extensively studied [1]. The phenomenon occurs when the 2′-OH group nucleophilically attacks the 3′-phosphate, leading to a cyclic 2′,3′-phosphate via a phosphorane, which is the key intermediate in RNA autohydrolysis (Figure 1a). Subsequently, the intermediate is hydrolysed in aqueous solution to 2′- and 3′-phosphate. Under acidic or basic conditions, RNA autohydrolysis can be accelerated a million-fold compared to spontaneous hydrolysis at neutral pH [15].
If there are no gaps in the PEG coating, it can be assumed that the entire mRNA will also remain intact for at least 5 days at 2°C to 8°C. Higher temperatures can shorten this time to a few hours. In the meantime, however, shelf lives have been extended and cooling requirements further reduced [16] and the 5 days has been increased to 30. The manufacturer is thus likely to have further improved the stabilisation of LNPs. It was worth investigating whether the stabilising PEG coating might show differences in quality due to the production process. Light-optical microscopic examinations of mRNA vaccines showed very unusual crystal-like impurities whose concentration increases with storage time. These are platelet-shaped geometric structures that show a strong resemblance to rhomboid cholesterol crystals [17].
Figure 3: Solid and liquid crystals of Cholesterol observed with polarised light microscopy: (d) tube-like crystal fractures (e) typical ChM crystals with angles of 79.2° and 100.8° and often notched corners.
Optical microscopy
In the meantime, there are numerous microscopic analyses of mRNA vaccines (concentrated and diluted) in BF (bright field) and DF (dark field) that show similar results. Researchers have reported observing unusual and unidentifiable geometric impurities next to the LNPs in the vaccine samples. Observations with a DF microscope revealed the following images for Moderna vaccines:
Figure 4: mRNA vaccine from the manufacturer Moderna stored at -18° C. and magnified 1000 times in the DF microscope
Crystallisation occurs more frequently during storage at room temperature. The milky cloudiness of Pfizer/Biontech Comirnaty is very likely to be related to crystallisation. This was also confirmed by simple slit-lamp examinations of originally sealed vaccine doses. Under the dark-field microscope, this becomes clearer and this phenomenon has also been documented with video recordings.
Figure 5: mRNA vaccine from Pfizer/Biontech stored at room temperature and 1000x magnification in a DF microscope.
A comparison with an aqueous solution of solid cholesterol (brand: Fisher Chemical C/5360/48) in the DF microscope clearly shows similar crystal structures to those observable in the vaccines:
Figure 6: Cholesterol crystals in aqueous solution
Investigations of dried inoculation samples on silicon carriers in the reflected light microscope and in the SEM (scanning electron microscope) showed further crystallisation phenomena on crystalline surfaces such as Si due to the inoculant-specific buffer and salt solutions:
Figure 7: Crystal formation of mRNA-1273 (Moderna) in the reflected light microscope (top) and in the scanning electron microscope (bottom)
Scanning Electron Microscopy (SEM)
Closer SEM examination of dried samples of the Moderna vaccine in the marginal areas show the crystallisation of larger platelet-like structures. Examination of these crystals with the electron beam then showed a clear NaCl signal in the EDS:
Figure 8: NaCl crystal platelets in dried mRNA-1273 in the scanning electron microscope and their EDS spectrum
Figures 4, 5 and 6 illustrate the appearance of solid cholesterol-containing crystals and optical textures of liquid crystals. After drying, filaments were observed that can be longer than 10 µm. In the liquid state, arch-like and occasionally spiral-like crystals are found. Tubular arrangements are also typical (Fig. 9. Plate-like cholesterol crystals-crystals usually occur with angles of 79.2° and 100.8° and often a notched corner. The precipitating liquid crystals tend to cluster (Fig.5) and usually form 1-5 µm crystallites, which can be called aggregates.
Figure 9: Tubular Cholesterol crystals in the SEM
During the drying process, the salts of the buffer solution also crystallise on existing lipid crystals and envelop them. Figure 10 shows a rectangular NaCl crystal that has an inner substructure with rounded corners indicating lipid crystals.
Figure 10: SEM NaCl crystal with Cholesterol embedding
The following questions arose from the optical observations given here as examples.
1. which material changes limit the lifespan of mRNA vaccines and can these be shown with simple measurement methods?
2. are material changes also detectable in certain batches due to the manufacturing process and how do they affect the safety profile?
It was explained above why nano-lipids are coated with hydrophilic PEG for stabilisation. Reference was also made to the combination with DSPC and cholesterol. A mass spectrometric procedure can be used to characterise the PEG coating. For this purpose, we made use of a Bruker MALDI time-of-flight mass spectrometer from one of the local universities.
Mass spectrometry
From an analytical viewpoint, PEG-containing samples are suitable for conventional spectroscopic methods such as matrix-assisted laser desorption ionisation time-of-flight mass spectrometry (MALDI-TOF MS) [18, 19].
The PEG samples were consequently prepared according to a standard protocol for MS measurement. MALDI-TOF mass spectra were recorded using the reflectron mode on an Ultraflex-TOF-TOF mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany). The serial instrument was equipped with a 337 nm, 50 Hz N2 laser. The peak resolution achieved was between m/z 250 - 950, or 1000 - 4000, depending on the range investigated. If the measurement is restricted to the mass range defined by the calibrant (m/z 3000-4000), more than 95 % of the peaks were measured with errors below 5 ppm.
For all the Comirnaty batches investigated, the material was extracted from previously sealed vials. The task was to identify the "flyable" components of the solid fractions and to examine them for qualitative differences. The measurement allows, for example, conclusions to be drawn about the number of ethylene oxide units (n) and the structure of the end group R of ALC-0159. Information is also obtained about the quality of ALC-0135.
Figure 11: TOF-MS fragmentation of RNA leads to the same cyclic phosphate intermediate that is formed during RNA autohydrolysis.
The fragmentation of RNA leads to 2′,3′-cyclic phosphates. The c- fragments formed in this process have the same m/z ratio as the cyclic phosphate intermediate that is formed during RNA autohydrolysis [18]. Measurements of different batches showed relatively strong differences in fragmentation at m/z=328.
Figure 12 shows spectra of different batches with different c-fragmentation. High relative proportions of c-fragments (EP2166) possibly indicate high proportions of intact mRNA. This observation also corresponds to the following observations for PEG.
Figure 12: MS of four batches of Comirnaty. m/z=328 differences indicate different mRNA loadings of LNPs.
The splitting at m/z=766 and m/z=764 of ALC-0315 may be related to the substitution of two hydrogens by a C=C double bond. It remains to be established whether this can be attributed to the MALDI methodology or to the manufacturing quality. Also, the ALC-0315 intensity agrees with the following PEG results.
The mass ranges m/z 1000 - 4000 show the PEG polymer distributions of the analysed batches. In Fig. 12 these differences become obvious and require explanation. First, shortened polymer chains could be generated either during MALDI analysis by in-source fragmentation or during sample preparation. More likely, however, are incomplete reactions leading to the presence of by-products (unreacted starting materials, possibly simply substituted PEG or unexpected modification of the end group). These products could be stable in the remaining synthesis steps or react further to yield increasingly complex mixtures with ions detected over a wide mass range.
Figure 13: PEG range m/z 2000-3500 shows batch-dependent polymerisation results
If we then summarise the measured PEG polymer distributions in terms of the number of ethylene glycol units, we can interpret a shifted distribution of ethylene glycol units towards a higher degree of polymerisation as a possible incomplete polymerisation of the LNP surface.
Figure 14: Overview PEG polymerisation
Figure 15: Incomplete PEG polymerisation of the LNP surface
In the next step, we asked ourselves whether these interpreted PEG inhomogeneities could have an influence on side effects (ADR = Adverse Reactions) post-injection. Considering the strong dependence of LNPs and mRNA on the PEG stabilisation mechanism, this is a legitimate question. Batch-specific vaccine adverse reactions can be found at [20] in "How Bad is My Batch". The measurements indicated a significant statistical correlation between the maximum of the distribution (Fig. 14) of ethylene glycol units (Max Degree of Polymerisation) and the ADRs from the database [20].
Figure 16: Relationship between PEG polymerisation and vaccination side effects
Consequently, we first hypothesise that a shorter-chain and therefore more homogeneous PEG coating of the LNP surface can stabilise the vaccine for a long time and so prevent its disintegration before its transport to unintended bodily organs. Longer-chain polymerisation and therefore a more inhomogeneous coating are conversely an insufficient protection against rapid decay of the LNPs and destroy their function as a protective mRNA transporter. With the disintegration of the LNPs, cholesterols and cationic lipids are also released, which can form crystals. Further studies are needed in this area, especially accurate tests on animals, to learn more about the effects of PEG, cationic lipids and cholesterols.
References
[1] K. Greis, C. Kirschbaum, M. I. Taccone, M. Götze, S. Gewinner, W. Schöllkopf, G. Meijer, G. von Helden, K. Pagel, Angew. Chem. Int. Ed. 2022, 61, e202115481;
[2] Michael D. Buschmann, Manuel J. Carrasco, Suman Alishetty, Mikell Paige, Mohamad Gabriel Alameh, Drew Weissman,Vaccines (Basel) 2021 Jan; 9(1): 65. Published online 2021 Jan 19. doi: 10.3390/vaccines9010065, PMCID: PMC7836001
[3] Behlke MA., Oligonucleotides. 2008 Dec;18(4):305-19. doi: 10.1089/oli.2008.0164. PMID: 19025401
[4] Choi J., Indrisiunaite G., DeMirci H., Ieong K.-W., Wang J., Petrov A., Prabhakar A., Rechavi G., Dominissini D., He C., et al., Nat. Struct. Mol. Biol. 2018;25:208–216. doi: 10.1038/s41594-018-0030-z
[5] Lorenz C., Fotin-Mleczek M., Roth G., Becker C., Dam T.C., Verdurmen W.P.R., Brock R., Probst J., Schlake T., RNA Biol. 2011;8:627–636. doi: 10.4161/rna.8.4.15394.
[6] Diken M., Kreiter S., Selmi A., Britten C.M., Huber C., Türeci Ö., Sahin U., Gene Ther. 2011;18:702– 708. doi: 10.1038/gt.2011.17.
[7] Oberli M.A., Reichmuth A.M., Dorkin J.R., Mitchell M.J., Fenton O.S., Jaklenec A., Anderson D.G., Langer R., Blankschtein D., Nano Lett. 2017;17:1326–1335. doi: 10.1021/acs.nanolett.6b03329
[8] Pardi N., Hogan M.J., Naradikian M.S., Parkhouse K., Cain D.W., Jones L., Moody M.A., Verkerke H.P., Myles A., Willis E., et al., J. Exp. Med. 2018;215:1571–1588.
doi: 10.1084/jem.20171450
[9] COVID-19 Vaccine Emergency Use Authorizations (EUAs), EUA letter of authorization, https://www.fda.gov/media/144412/download
[10] Clinical Trial of a COVID-19 Vaccine in Healthy Adults,
https://www.modernatx.com/sites/default/files/mRNA-1273-P301-Protocol.pdf
[11] Fact Sheet for Healthcare Providers Administering Vaccine,
https://www.fda.gov/media/144637/download
[12] Pfizer-BioNTech Fact Sheets and FAQs, https://www.fda.gov/media/144413/download [13] Gómez G, Pikal MJ, Rodríguez-Hornedo N., Pharm Res. 2001 Jan;18(1):90-7. doi: 10.1023/a:1011082911917. PMID: 11336359.
[14] Tang X, Pikal MJ., Pharm Res. 2004 Feb;21(2):191-200. doi:
10.1023/b:pham.0000016234.73023.75. PMID: 15032301
[15] Emilsson GM, Nakamura S, Roth A, Breaker RR., RNA. 2003 Aug;9(8):907-18. doi: 10.1261/rna.5680603. PMID: 12869701; PMCID: PMC1370456
[16] EMA press office, https://www.ema.europa.eu/en/news/more-flexible-storage-conditions biontechpfizers-covid-19-vaccine
[17] D Q Wang, M C Carey, Journal of Lipid Research, 1996, vol 37(3), 606-630, ISSN 0022- 2275 [18] Christine Enjalbal et al, MALDI-TOF Journal of the American Society for Mass Spectrometry, 2005, vol 16(5), 670-678, ISSN 1044-0305
[19] Na, D.H., Youn, Y.S. and Lee, K.C. (2003), Rapid Commun. Mass Spectrom., 17: 2241- 2244. https://doi.org/10.1002/rcm.1175
[20] Batch codes and associated deaths, disabilities and illnesses for Covid 19 Vaccines, https://howbad.info/
Analytical report - Inorganic analysis of the vaccines
Samples:
• 3 (opened) vials of Moderna with residues of 1-3 g liquid each.
• 8 (opened) vials of Biontech with very small residues. All liquids were combined and yielded approx. 2 g liquid
Analytical device: inductively coupled plasma (ICP). In ICP, the atoms of an element are excited in order to emit radiation. This radiation is detected and the content of the respective element can be determined in comparison to standard solutions. Unfortunately, a high amount of sample material is needed for the ICP measurement compared to other analytical methods. Therefore, the samples had to be diluted as described below (or combined and diluted at BioNTech) in order to obtain a reasonable measurement at all.
Since only dissolved elements can be recorded in the ICP measurement (dissolving the particles, platelets, threads, etc.), the sample was mixed with HNO3 and then heated (digestion).
Sample preparation: The samples were measured into clean 10 ml glass flasks and mixed with 0.8 ml conc. nitric acid. Then heated for approx. 30 min at 85°C on the hotplate and then filled up to the calibration mark with double-distilled water. Water was added up to the calibration mark.
A blank sample (digestion without sample) was also prepared in order to exclude the possibility of impurities introduced by me or the HNO3.
A digestion was prepared from each Moderna vial: M1; M2; M3
The BioNTech vials were all combined into one digestion: BT
ICP measurement: For the elements listed in the table, the ICP was calibrated with a 4-point straight line. This means that one blank value and 3 standards (ß(E)= 0.6; 1; 2 mg/l) were measured for each element. For antimony another standard series was calibrated ß(Sb)= 1; 2; 5 mg/l.
With the ICP, only those elements were analysed that were also searched for using standard solutions. For several reasons, elements that are not listed here were not tested for.
The values are converted to the original solutions and not to the dilution.
The results of all elements, with the exception of antimony, are below the corresponding detection limit. The detection limit results from the sample quantity (unfortunately high LOD because there is so little of the sample material); the intensity of the signal of the particular element in the ICP and overall contamination in the laboratory (double-distilled water; laboratory equipment; ICP). I had to set the detection limit for silicon at such a high level because I deal a lot with substances in my laboratory that contain silicon as a main component and there is therefore a risk of contamination. The vaccine vials and the laboratory equipment are also made of glass and can therefore deliver elevated Si values.
A signal is clearly detectable for antimony (Sb). To rule out interference from other elements, antimony was measured at three different wavelengths, all of which provide the same result. The blank value runs on baseline (indicating that contamination on my part is improbable).
I did several measurements of the solutions (also before and after the weekend) and found that the longer the digestion solutions stand, the smaller the antimony value. One can observe that with time the lipids contained in the vaccine form larger white flakes in the nitric acid solution. It is possible that the antimony is absorbed by these flakes and I consequently obtain lower and lower values as this process progresses.
It must be stated that the nitric acid digestion is far from ideal. A better solution would be one in which the lipids are also dissolved (possibly using a mixture of water and organic solvent). Unfortunately, I had no scope for this owing to the small amount of sample.
If the antimony value is significant from the point of view of the working group, further analyses would be necessary. My result is an indication that antimony could be present in the Moderna sample, as a clear signal can be identified at different antimony wavelengths.
To verify the result, further analyses would have to be carried out with unopened vials and further digestion procedures.






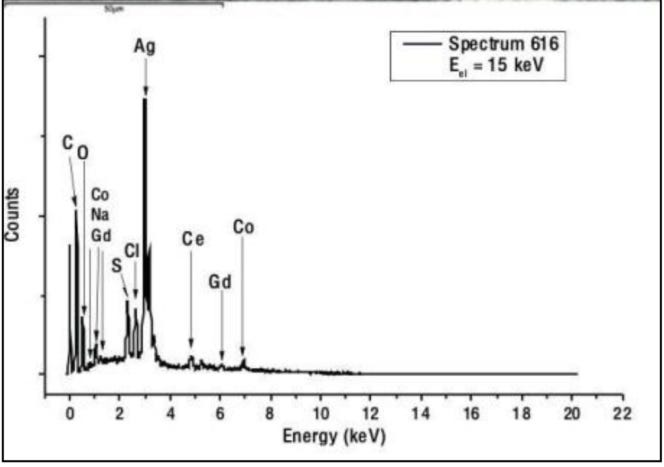
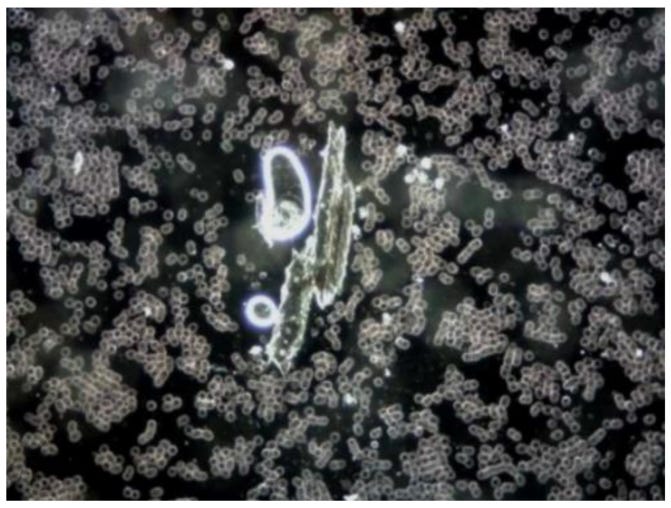





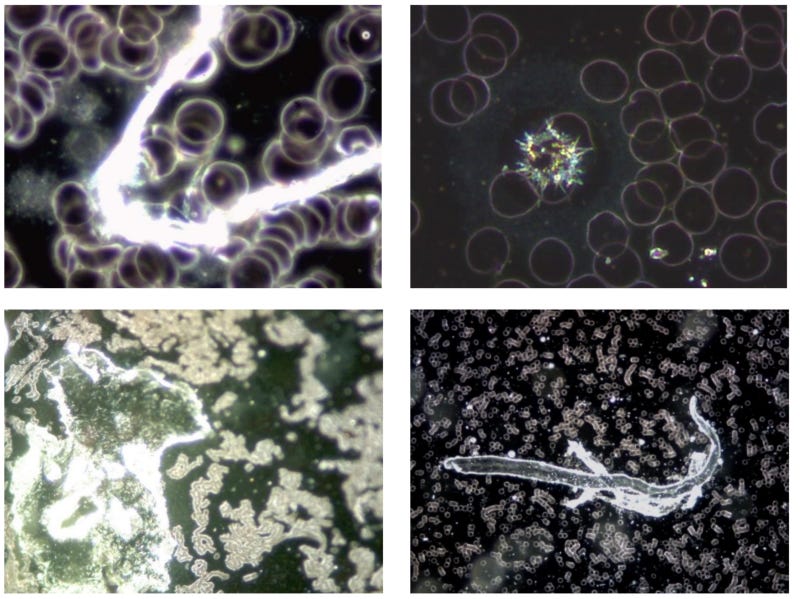
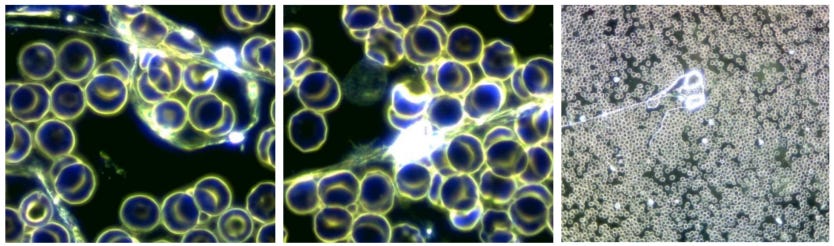








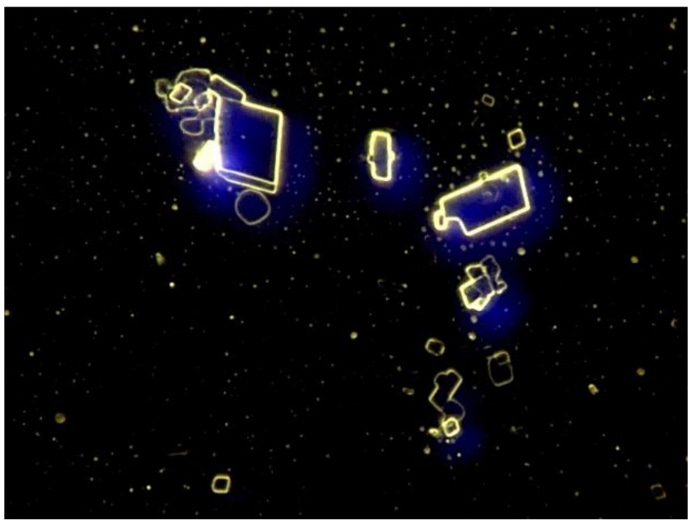



















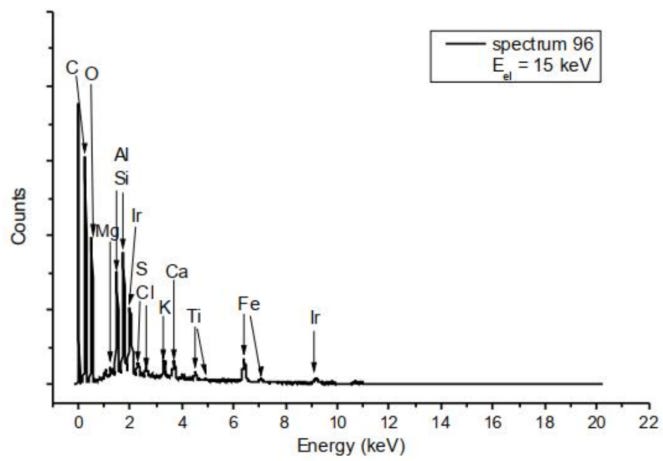





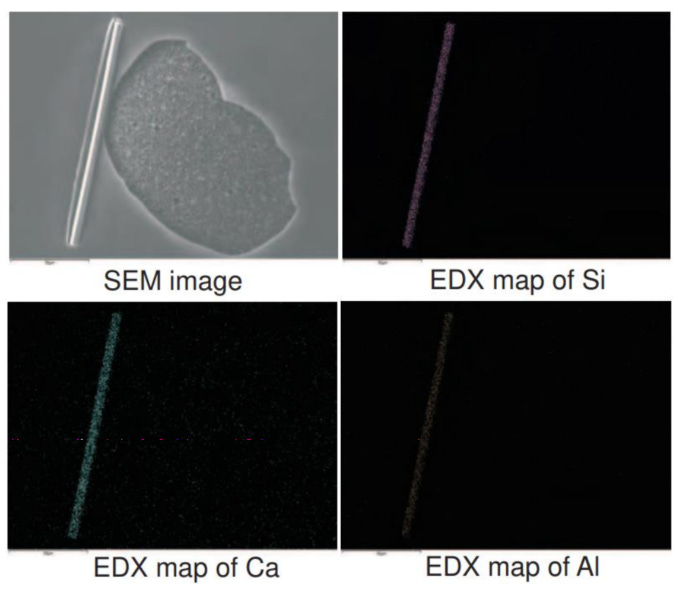














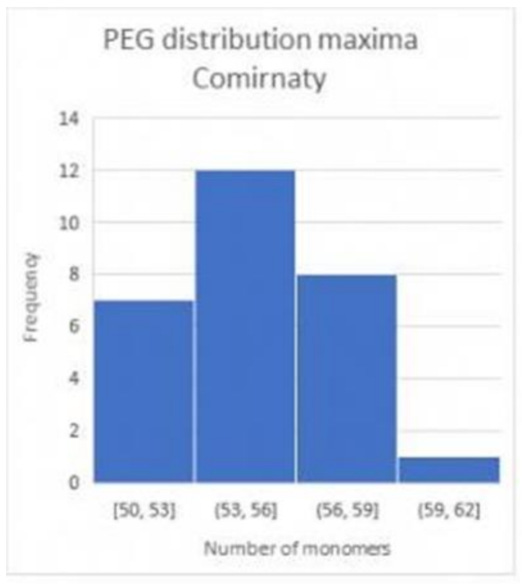




We have VAERS Germany data: https://www.vaersaware.com/c19-only
I was actually trying to find the expiration date on Lot# 1F1010A . Might you know what that is?